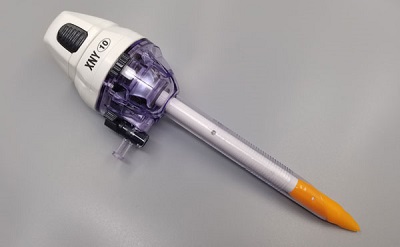
Use of trocar
• Remove the device from the package in a sterile manner. To avoid injury, do not turn the device over into the sterile area
• Insert the pneumoperitoneum needle into the abdomen 1cm above or below the umbilicus and connect the catheter of the pneumoperitoneum machine to the valve of the pneumoperitoneum needle. Turn the valve to open and inject carbon dioxide gas into the abdominal cavity to establish a pneumoperitoneum.
• Remove the puncture sleeve and core from the aseptic package, insert the puncture core into the puncture sleeve, the puncture core tip is completely exposed, assemble the puncture device, and check whether the performance of the product is normal.
• The choke valve on the puncture device in the factory package is in an open position. Close the choke valve before use. When the choke valve stem is parallel to the puncture sleeve, the choke valve is in the closed position.
• Pneumoperitoneum is established by pressing down the outer tube of the puncture device against the body wall to produce a circular mark, and then appropriately expanding the mark. A skin incision large enough to accommodate the cannula is made in the abdominal cavity to accommodate the cannula entry
• During the puncture, the top of the puncture core should be placed in the palm, and two fingers should be placed on the ears of the casing separately, and the puncture can be achieved with a slight grip
Taboo of using trocar used in laparoscopic surgery:
• This product cannot be used in newborn patients, pregnant women should use with caution; Other patients who are not suitable for endoscopic surgery are contraindicated. This product is a disposable product, and repeated sterilization and reuse are strictly prohibited.
Using trocar that matters needing attention:
• Please read the instructions and check the package integrity before use. If there is any damage or expiration date, please stop using the product and sterilize it before leaving the factory
• This product is only for one-time use, and repeated use is prohibited; The disposal of waste shall be carried out in accordance with relevant laws and regulations
• The diameter of minimally invasive endoscopic instruments may be different due to different manufacturers. When minimally invasive instruments and accessories from different manufacturers are used in the same minimally invasive surgery, it is necessary to verify whether these instruments and accessories are compatible before surgery
• If a puncture device of 10mm or 12mm is used, the sealing cap can be rotated when large tissue needs to be removed from the cavity at the end of the operation, and the large tissue needs to be removed from the puncture cannula. After the operation, an endoscope should be used to confirm whether there is bleeding in the abdominal wall and abdominal cavity. The cannulation is then slowly removed from the insertion end by a trained health care worker,
• Puncture should be slow to avoid accidental injury to internal organs
• Disposal of the product after use shall be handled in accordance with relevant local laws and regulations.
Post time: Oct-13-2021