Disposable Laparoscopic Linear Cutter Stapler and Components part 4
(Please read the instruction manual carefully before installing and using this product)
VIII. Laparoscopic Linear Cutting Stapler maintenance and maintenance methods:
1. Storage: Store in a room with a relative humidity not greater than 80%, well ventilated, and no corrosive gases.
2. Transportation: The packaged product can be transported with normal tools. During transportation, it should be handled with care and avoid direct sunlight, violent collision, rain and gravity extrusion.
IX. Laparoscopic Linear Cutting Stapler expiration date:
After the product is sterilized by ethylene oxide, the sterilization period is three years, and the expiration date is shown on the label.
X.Laparoscopic Linear Cutting Stapler accessories list:
none
Precautions and warnings for XI. Laparoscopic Linear Cutting Stapler:
1. When using this product, aseptic operation specifications should be strictly followed;
2. Please check the packaging of this product carefully before use, if the blister packaging is damaged, please stop using it;
3. This product is sterilized by ethylene oxide, and the sterilized product is for clinical use. Please check the disk indicator on the sterilization packaging box of this product, “blue” means that the product has been sterilized and can be directly used clinically;
4. This product is used for one operation and cannot be sterilized after use;
5. Please check whether the product is within the validity period before use. The sterilization validity period is three years. Products beyond the validity period are strictly prohibited;
6. The laparoscopic cutting assembly produced by our company must be used in conjunction with the corresponding type and specification of the disposable laparoscopic linear cutting stapler produced by our company. See Table 1 and Table 2 for details;
7. Minimally invasive operations should be performed by persons who have received sufficient training and are familiar with minimally invasive techniques. Before performing any minimally invasive surgery, the medical literature related to the technique, its complications and hazards should be consulted;
8. The size of minimally invasive equipment from different manufacturers may be different. If minimally invasive surgical instruments and accessories produced by different manufacturers are used in one operation at the same time, it is necessary to check whether they are compatible before the operation;
9. Radiation therapy before surgery may cause tissue changes. For example, these changes may cause tissue thickening beyond what is specified for the chosen staple. Any treatment of a patient prior to surgery should be carefully considered and may require changes in surgical technique or approach;
10. Do not release the button until the instrument is ready to fire;
11. Be sure to check the safety of the staple cartridge before firing;
12. After firing, be sure to check the hemostasis at the anastomotic line, check whether the anastomosis is complete and whether there is any leakage;
13. Ensure that the tissue thickness is within the specified range and that the tissue is evenly distributed within the stapler. Too much tissue on one side can cause poor anastomosis, and anastomotic leakage may occur;
14. In the case of excess or thick tissue, attempting to force the trigger may result in incomplete sutures and possible anastomotic rupture or leakage. In addition, instrument damage or failure to fire may occur;
15. One shot must be completed. Never partially fire the instrument. Incomplete firing may result in improperly formed staples, incomplete cut line, bleeding and leakage from the suture, and/or difficulty removing the instrument;
16. Be sure to fire to the end to ensure that the staples are correctly formed and the tissue is cut correctly;
17. Squeeze the firing handle to expose the cutting blade. Do not press the handle repeatedly, which will cause damage to the anastomosis site;
18. When inserting the device, ensure that the safety is in the closed position to avoid inadvertent activation of the firing lever, resulting in accidental exposure of the blade and premature partial or full deployment of the staples;
19. The maximum firing times of this product is 8 times;
20. Using this device with anastomotic line reinforcement materials may reduce the number of shots;
21. This product is a single-use device. Once the device is opened, no matter whether it is used or not, it cannot be sterilized again. Make sure to lock the safety lock before handling;
22. Safe under certain conditions of nuclear magnetic resonance (MR):
·Non-clinical tests show that implantable staples with the material grade of TA2G can be used for MR conditionally. Patients can safely be scanned immediately after staple insertion in the following situations:
·The range of static magnetic field is only between 1.5T-3.0T.
·The maximum spatial magnetic field gradient is 3000 gauss/cm or below.
·The largest reported MR system, scanning for 15 minutes, the whole body average absorption ratio (SAR) is 2 W/kg.
·Under scanning conditions, the maximum temperature rise of staples is expected to be 1.9°C after scanning for 15 minutes.
Artifact information:
When non-clinically tested using gradient echo pulse sequence imaging and a static magnetic field 3.0T MR system, staples caused artifacts approximately 5 mm from the implant site.
23. See the label for the production date;
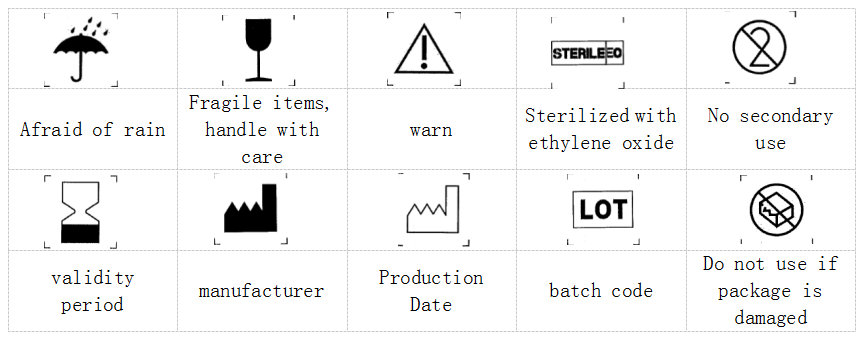
24. Explanation of graphics, symbols and abbreviations used in packaging and labels:
Related ProductsPost time: Jan-20-2023