Disposable vacuum blood collection tube’s standard
11 scope
This standard specifies the product classification, technical requirements, test methods, inspection rules, information provided and identification of additives of disposable vacuum blood collection tubes (hereinafter referred to as blood collection tubes).
This standard is applicable to disposable vacuum blood collection tubes.
12 normative references
The clauses in the following documents become the clauses of this standard by reference. For dated reference documents, all subsequent amendments (excluding the contents of Corrigendum) or revisions are not applicable to this standard. However, all parties who reach an agreement according to this standard are encouraged to study whether the latest version of these documents can be used. For undated references, the latest version is applicable to this standard.
GB / t191-2008 pictorial signs for packaging, storage and transportation
GB 9890 medical rubber stopper
YY 0314-2007 disposable human venous blood sample collection container
WS / t224-2002 vacuum blood collection tube and its additives
Yy0466-2003 medical devices: symbols for labeling, marking and providing information of medical devices
13 product structure classification
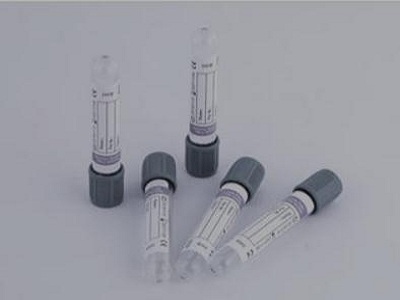
13.1 the structure of typical blood collection vessels is shown in Figure 1
1. Containers; 2. Stopper; 3 cap.
Note 1: Figure 1 shows the typical structure of the blood collection vessel. As long as the same effect can be achieved, other structures can also be used
Figure 1 example of typical blood collection vessel
13.2 product classification
3.2.1 classification by use:
Table 1 Classification of blood collection vessels (by additive)
Sn name Sn name
1 common tube (serum tube or empty tube) 7 heparin tube (heparin sodium / heparin lithium)
2 coagulation promoting tube (quick coagulation tube) 8 blood coagulation tube (sodium citrate 1:9)
3 separation gel (separation gel / coagulant) 9 hemoprecipitation tube (sodium citrate 1:4)
4 blood routine tube (edtak) 10 blood glucose tube (sodium fluoride / potassium oxalate)
5 blood routine tube (edtak) 11 sterile tube
6 blood routine tube (edtana) 12 pyrogen free tube
3.2.2 according to nominal capacity: 1ml, 1.6ml, 1.8ml, 2ml, 2.7ml, 3ml, 4ml, 5ml, 6ml, 7ml, 8ml, 9ml, 10ml, 11ml, 12ml, 15ml, etc.
Note: special specifications can be customized according to customer requirements.
14 technical requirements and experimental methods
14.1 technical requirements
4.1.1 dimensions
4.1.1.1 the size of blood collection tube (tube size) is expressed by outer diameter and length:
Table 2 size of blood collection vessel (unit: mm)
No. outer diameter * length No. outer diameter * length No. outer diameter * length
1 13*100 5 12.5*95 9 12*75
2 13*95 6 12.5*75 10 9*120
3 13*75 7 12*100 11 8*120
4 12.5*100 8 12*95 12 8*110
Note: the allowable error of outer diameter is ± 1mm, and the allowable error of length is ± 5mm.
The size of blood collection tube can be customized according to customer requirements.
4.1.2 appearance
4.1.2.1 the blood collection vessel shall be transparent enough to clearly observe the contents during visual inspection.
4.1.2.2 the plug shall be clean in appearance, free of crack or defect, obvious flash and obvious mechanical impurities.
4.1.2.3 it is recommended that the color of the cap of blood collection tube should be specified in Table 1 of article 12.1 of yy0314-2007 standard.
Test method: observe with eyes.
4.1.3 tightness
It shall comply with Appendix C of yy0314-2007. The plug shall not be loosened during the container leakage test. The blood collection tube shall pass the leakage test.
Test method: conduct the test according to Appendix C of yy0314-2007.
Post time: Aug-22-2022